Elena & Fabrice's Web
Hydrogen
Hydrogen is the most common element in the Universe. Even ourselves, being made of about 70% of water, itself consisting of 66% hydrogen (the terminology hydrogen comes from making water), we are basically hydrogen. It is therefore an important object to understand in some details.
The Bohr model
Spectroscopy in the late 19th century discovered that the radiation from hydrogen consisted of spectral lines, i.e., the emission occurs at precise frequencies.
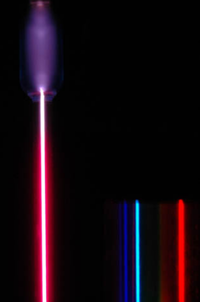
Empirically, it was found (by Rydberg) that the lines occurred at wavelengths following this formula:
$${\displaystyle {\frac {1}{\lambda _{\mathrm {vac} }}}=R_{\text{H}}\left({\frac {1}{n_{1}^{2}}}-{\frac {1}{n_{2}^{2}}}\right),}$$